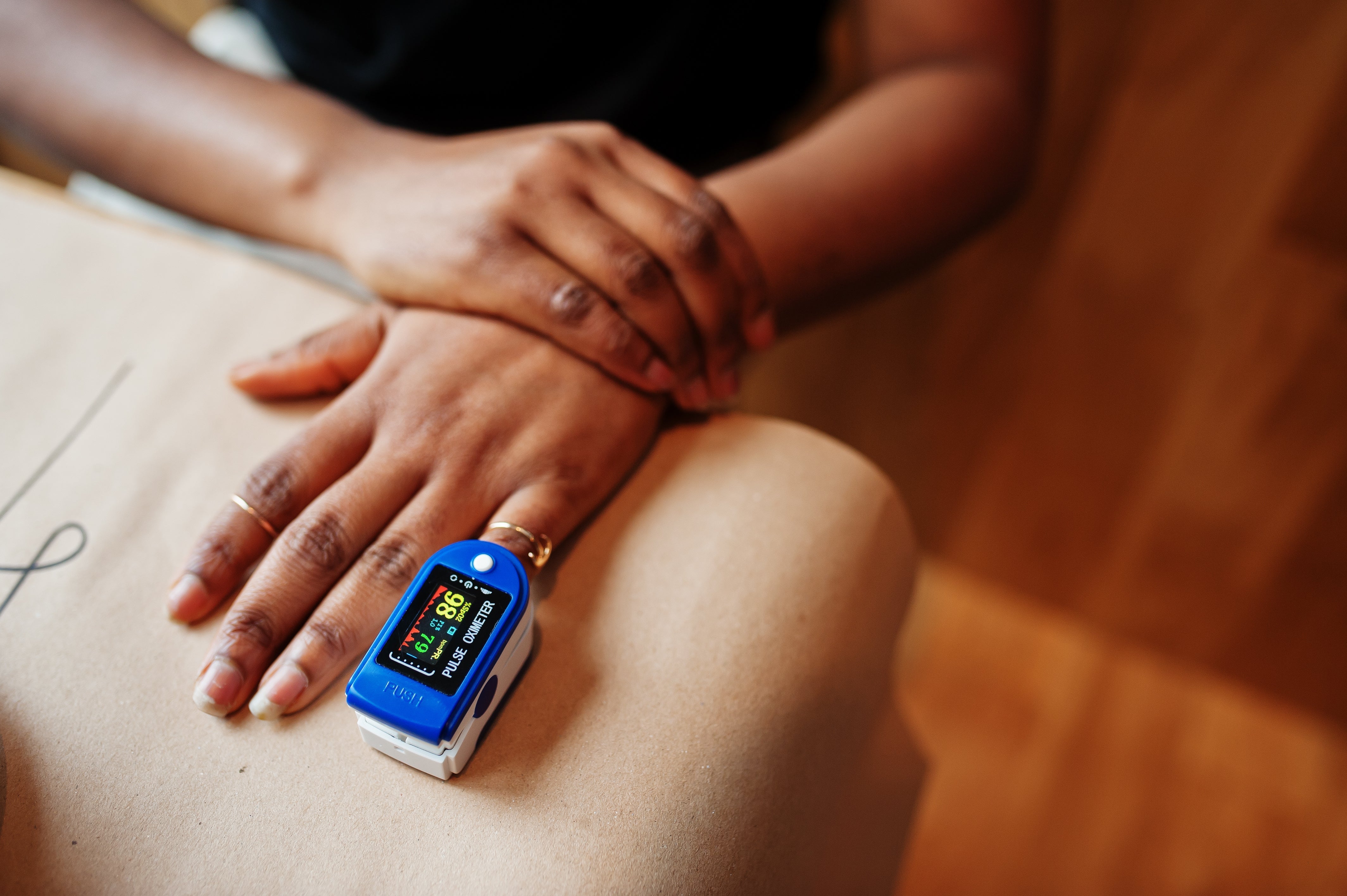
ECRI Examines Reliability of Pulse Oximeters on People with Varying Skin Pigmentation
Darker skin pigmentation can adversely influence pulse oximeter readings, leading to missed or delayed diagnoses and treatment. This health disparity has caused the U.S. Food and Drug Administration (FDA) to issue draft guidance on pulse oximeters, which are used to estimate the amount of oxygen in the blood, and are able to detect conditions such as sleep apnea and heart disease.
ECRI’s recent evidence analysis on pulse oximeters found that readings for individuals with darker skin might overestimate hemoglobin's light absorption, showing normal oxygenation levels despite hypoxemia. This inaccuracy raises the risk of undetected hypoxemia and adverse outcomes in hospitalized patients with darker skin pigmentation.
ECRI has a long history of evaluating medical devices and issued its first report on pulse oximeters in 1989. At an FDA advisory panel meeting earlier this year, Dr. Scott Lucas, ECRI’s vice president of device safety, explained the “significant health equity issue” associated with pulse oximeters. “The color of a patient’s skin should never degrade the quality or the effectiveness of tools that healthcare providers use to give lifesaving care,” Lucas said.
Studies Illustrate Health Disparities
Several recently published studies have also confirmed that these sensors are more inaccurate on darker skin tones. Among these studies was a New England Journal of Medicine report that showed darker skin tones receive more inaccurate readings than lighter ones. In Black patients, 11.7% of apparently normal readings were incorrect compared with 3.6% of readings in White patients.
This health technology issue also prompted more than 20 state attorneys general to send a letter to FDA in late 2023 urging for updated guidelines to include warnings that address pulse oximetry’s inaccuracy on darker skin. “It is unconscionable in a multi-racial, multi-ethnic nation with populations spanning the color, age, and medical vulnerability spectra that sub-optimal and potentially harmful diagnostic technology such as the pulse oximeter is not calibrated to adjust for skin color,” the letter states.
ECRI’s Recommendations
ECRI outlined three main recommendations for FDA to improve the quality of premarket studies and associated methods used to evaluate the performance of pulse oximeters.
- Pilot test the proposed Monk Skin Tone (MST) protocol to assess skin tone and skin variations across many patients. Train users on the tool, and evaluate whether they can use it consistently. Assess whether different users can get the same reading on the same patient, then address discrepancies through cognitive interviewing. Update relevant training programs.
- MST is typically performed by looking at the skin pigment on a patient’s forehead, but pulse oximeters are primarily placed on the finger. For many people, skin pigment differs between the face and hands. Identify MST at the pulse oximeter sensor location, the finger (which is consistent with FDA’s proposal for quantification of skin tone).
- For calculating Individual Typology Angle, choose colorimetry devices validated for reliable and consistent use across diverse users. Some device vendors may have completed testing that shows they can produce consistent readings. Without that validation data, conduct a pre-study validation trial to assess whether individuals use the colorimeters consistently, such as at the same angle and same pressure against the skin and whether different users get the same readings on a single patient.
ECRI’s evidence analysis concluded that, based on the published clinical studies, pulse oximeter readings from individuals with darker skin may overestimate the light absorption from hemoglobin and report normal peripheral oxygen saturation (SpO2) when the individuals are actually hypoxemic. Less accurate SpO2 measurements in hospitalized patients with darker skin pigmentation increases the risk of undetected (occult) hypoxemia and adverse clinical outcomes.
ECRI members may access this Clinical Evidence Assessment through ECRI’s web portal. Nonmembers may learn more about Clinical Evidence Assessment and request additional information.
